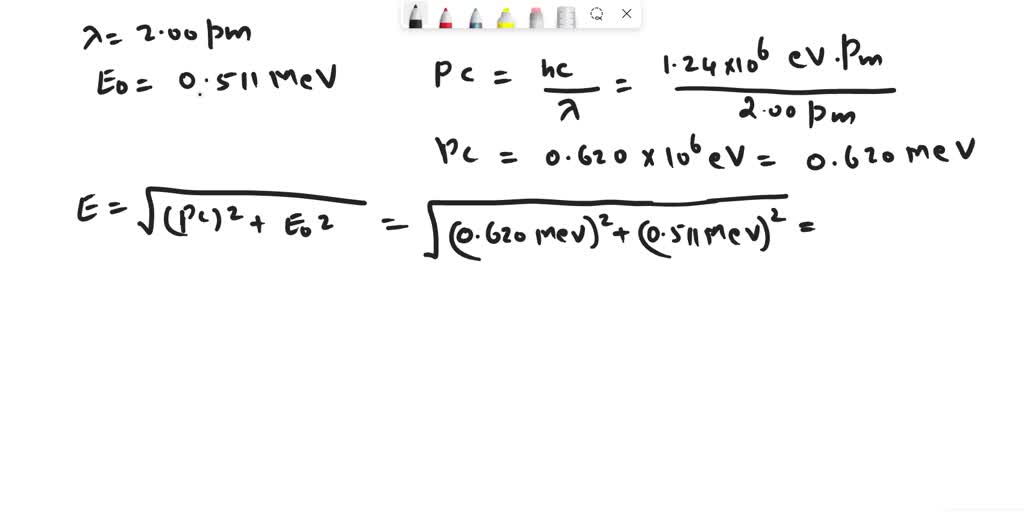
SOLVED: An electron has a de Broglie wavelength of 2.00 pm. Find its kinetic energy and the phase and group velocities of its de-Broglie waves.
The kinetic energy of an electron which is associatedwith de Broglie's wavelength 20 A is (1) 1.0 eV (2) 1.51 ev (3) 0.59 eV (4) 0.38 eV

Matter waves 1 Show that the wavelength of an electron can be expressed as where E is the energy in volts and in nm. - ppt download
Establish formula to find de-Broglie wavelength of electron, proton and α-particle and uncharged particles. - Sarthaks eConnect | Largest Online Education Community
Derive an expression for de Broglie wavelength of electrons. - Sarthaks eConnect | Largest Online Education Community

de Broglie wavelength of an electron after being accelerated by a potential difference of V volt - YouTube

If a proton and an electron have the same de Broglie wavelengths, which one is moving faster? - Quora

What is the de Broglie wavelength of an electron in the ground state of a hydrogen atom? - CBSE Tuts
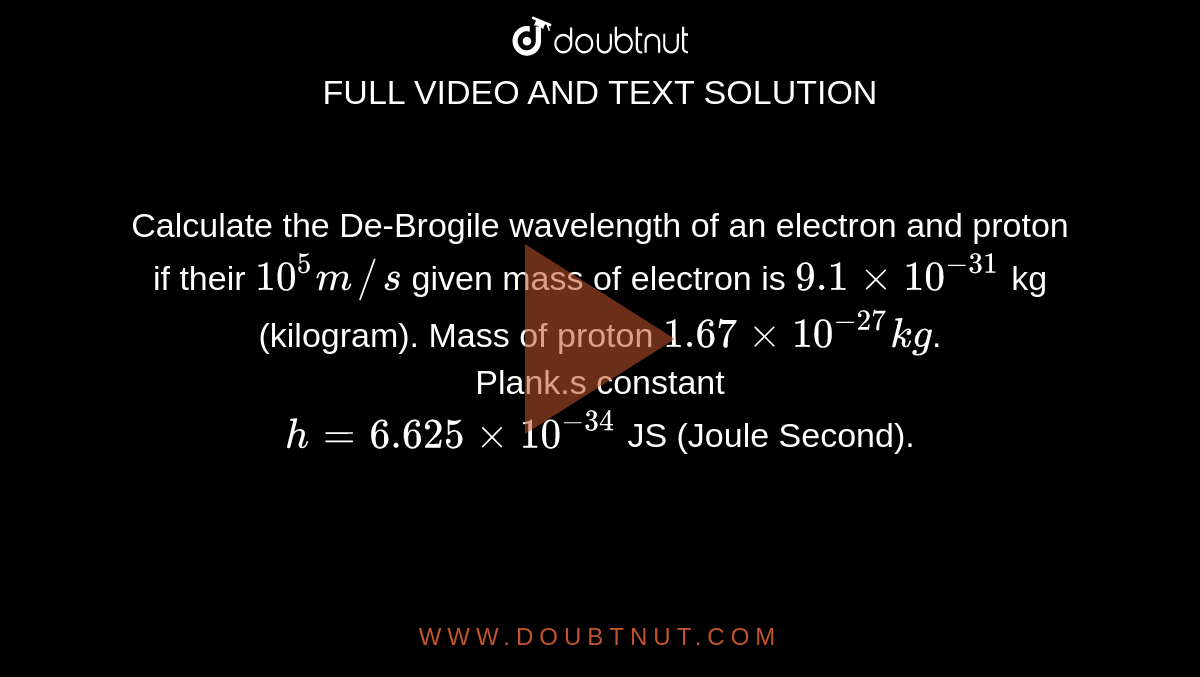
Calculate the De-Brogile wavelength of an electron and proton if their 10^5 m//s given mass of electron is 9.1 xx 10^(-31) kg (kilogram). Mass of proton 1.67 xx 10^(-27) kg. Plank.s constant
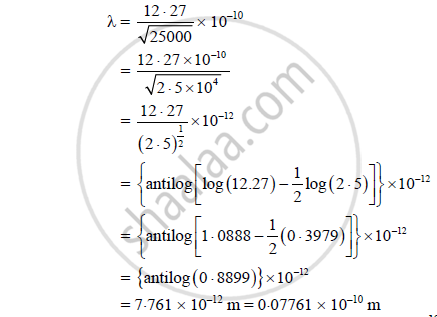
What is De Broglie Wavelength of an Electron Accelerated Through 25000 Volt? - Physics | Shaalaa.com

Calculate the de-Broglie wavelength of an electron (mass = 9.1 X ${{10}^{-31}}$ kg) moving at 1% speed of light (h = 6.63 X ${{10}^{-34}}$ kg ${{m}^{2}}$ ${{s}^{-1}}$ - CBSE Class 11 Chemistry - Learn CBSE Forum
![Show that de - Broglie wavelength of electron accelerated through V volt is nearly given by: lambda = [1550/V]^1/2 in A^∘ Show that de - Broglie wavelength of electron accelerated through V volt is nearly given by: lambda = [1550/V]^1/2 in A^∘](https://haygot.s3.amazonaws.com/questions/1764716_1741456_ans_29d59287cc9f42d59caf625f05e8c802.jpg)
Show that de - Broglie wavelength of electron accelerated through V volt is nearly given by: lambda = [1550/V]^1/2 in A^∘









